About us
- Solvatochromic Pyrene Analogs of Prodan Exhibiting Extremely High Fluorescence Quantum Yields in Apolar and Polar Solvents
- Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes
- Highly twisted N,N-dialkylamines as a design strategy for turn of typical aromatic hydrocarbons as steric environment-sensitive fluorophores
- Novel pyrene-based two-photon active fluorescent dye efficiently excited and emitting in the ‘tissue optical window (650–1100 nm)’
- Fluorescence Enhancement of Pyrene Chromophores Induced by Alkyl Groups through sigma-pi Conjugation: Systematic Synthesis of Primary, Secondary, and Tertiary Alkylated Pyrenes at the 1, 3, 6, and 8 Positions and Their Photophysical Properties
- Development of Laser Dyes to Realize Low Threshold in Dye-doped Cholesteric Liquid Crystal Lasers
Solvatochromic Pyrene Analogs of Prodan Exhibiting Extremely High Fluorescence Quantum Yields in Apolar and Polar Solvents
Chem. Eur. J. 2013 [DOI: 10.1002/chem.201301020]
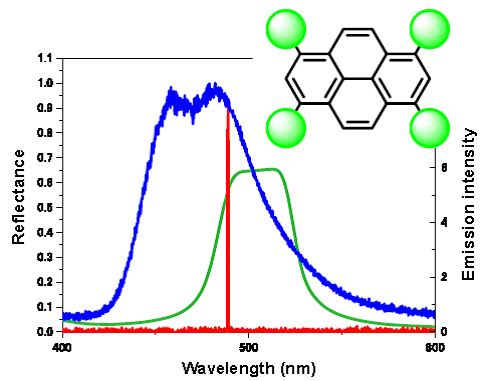
Solvatochromic dye: Novel pyrene analogs of Prodan exhibit outstanding photophysical properties, i.e., remarkably high fluorescence quantum yield (QY) in solvents ranging from apolar hexane to polar methanol (QY 0.88, 0.97, and 0.85 in hexane, dichloromethane, and methanol, respectively), that are accompanied by strong solvatochromism and large Stokes shifts. These properties have not been previously achieved in enormous solvatochromic dyes but are quite useful for emitting materials and imaging tools.
Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes
Sci. Rep. 2016 [DOI: 10.1038/srep18870]
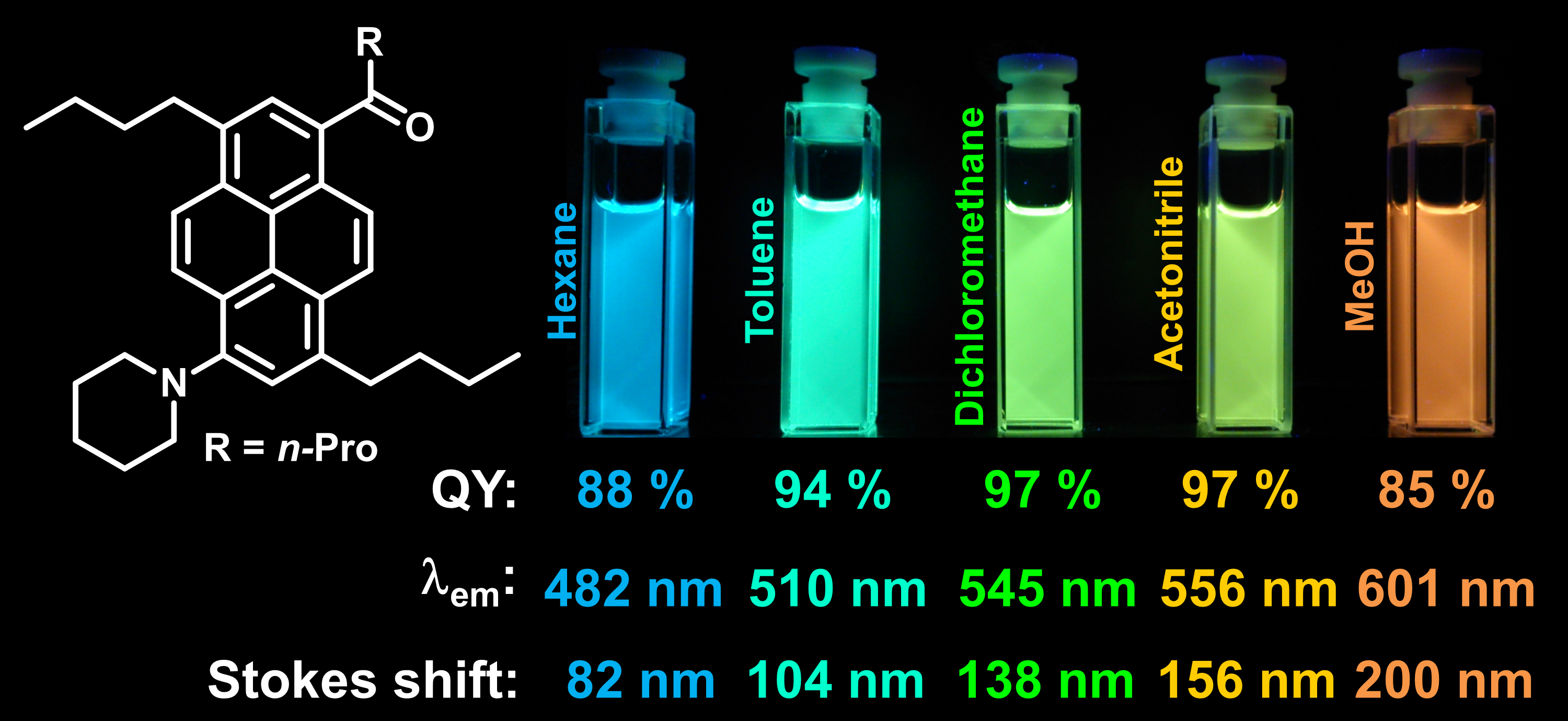
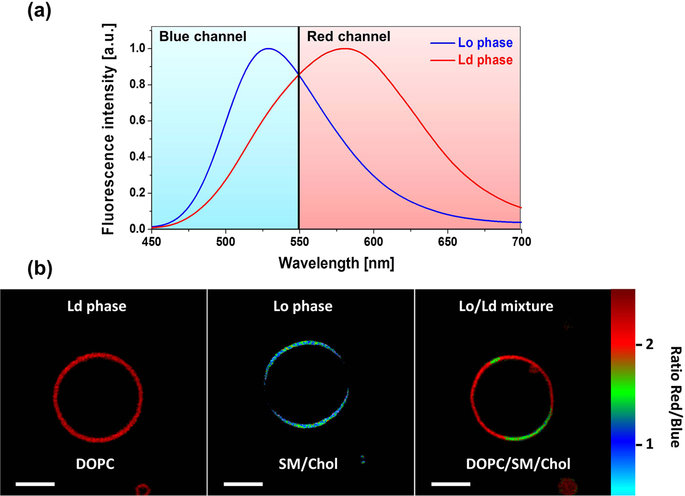
Imaging lipid organization in cell membranes requires advanced fluorescent probes. Here, we show that a recently synthesized push-pull pyrene (PA), similarly to popular probe Laurdan, changes the emission maximum as a function of lipid order, but outperforms it by spectroscopic properties. In addition to red-shifted absorption compatible with common 405 nm diode laser, PA shows higher brightness and much higher photostability than Laurdan in apolar membrane environments. Moreover, PA is compatible with two-photon excitation at wavelengths >800 nm, which was successfully used for ratiometric imaging of coexisting liquid ordered and disordered phases in giant unilamellar vesicles. Fluorescence confocal microscopy in Hela cells revealed that PA efficiently stains the plasma membrane and the intracellular membranes at >20-fold lower concentrations, as compared to Laurdan. Finally, ratiometric imaging using PA reveals variation of lipid order within different cellular compartments: plasma membranes are close to liquid ordered phase of model membranes composed of sphingomyelin and cholesterol, while intracellular membranes are much less ordered, matching well membranes composed of unsaturated phospholipids without cholesterol. These differences in the lipid order were confirmed by fluorescence lifetime imaging (FLIM) at the blue edge of PA emission band. PA probe constitutes thus a new powerful tool for biomembrane research.
Highly twisted N,N-dialkylamines as a design strategy for turn of typical aromatic hydrocarbons as steric environment-sensitive fluorophores
J. Am. Chem. Soc. 2016 [DOI: 10.1021/jacs.6b03749]
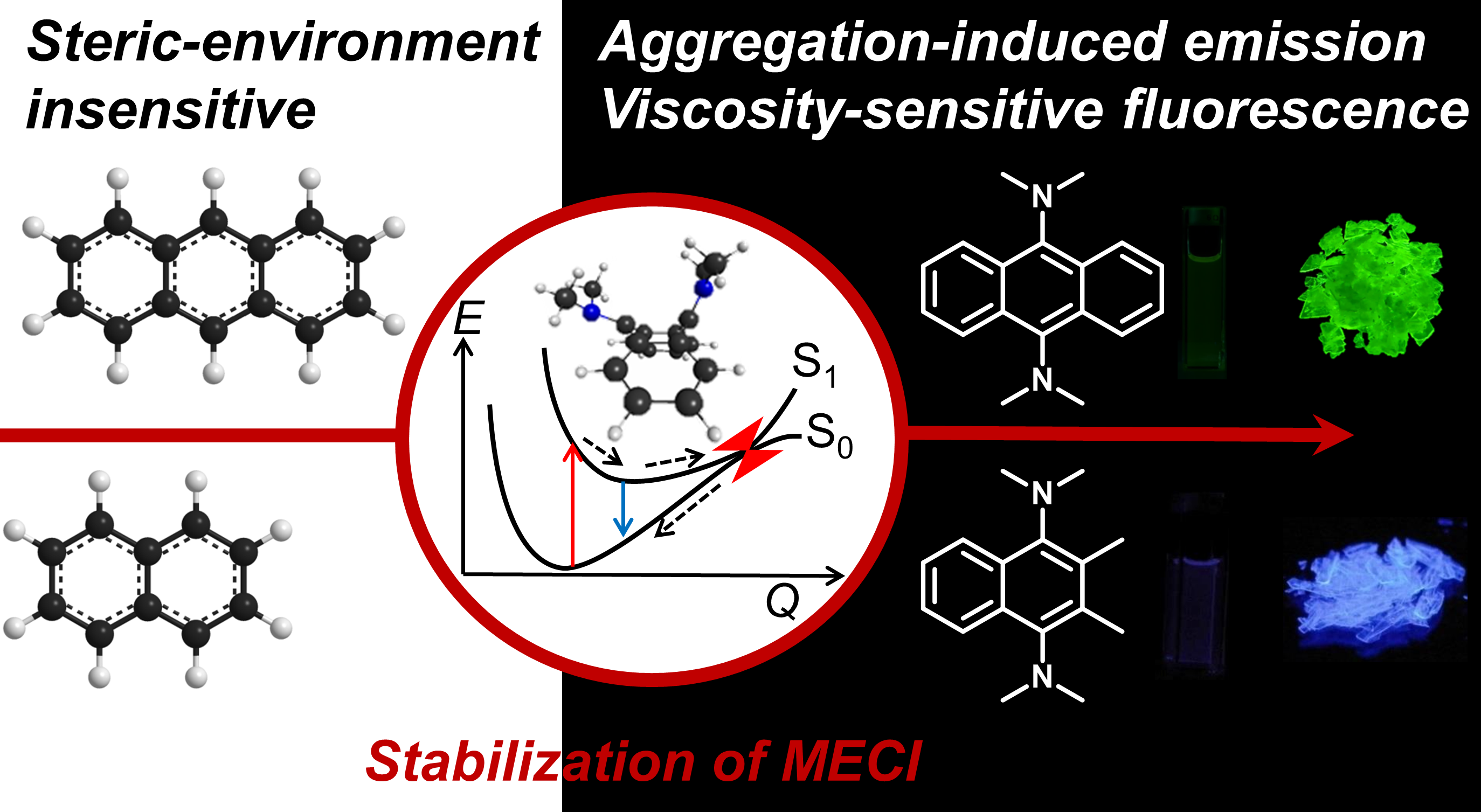
The steric-environment sensitivity of fluorescence of 9,10-bis(N,N-dialkylamino)anthracenes (BDAAs) was studied experimentally and theoretically. A new design strategy to tune simple aromatic hydrocarbons as efficient aggregation-induced emission (AIE) luminogens and molecular rotors is proposed. For a variety of BDAAs, prominent Stokes shifts and efficient solid-state fluorescence were observed. Calculations on BDAA-methyl suggested that in the ground state (S0) conformations, the pyramidal amine groups are highly twisted, so that their lone-pair orbitals cannot conjugate with the anthracene π orbitals. Fluorescence takes place from the S1 minima, in which one or both amine groups are planarized. The stability of the S1 excited state minima as well as destabilization of the S0 state is the origin of large Stokes shift. Experimental measurement of the non-adiabatic transition rate suggests that para disubstitution by dialkylamino (or strongly electron-donating) groups is a key for fast internal conversion. Minimum energy conical intersection (MECI) between S1 and S0 states was found to have a Dewar-benzene like structure. Although this can be reached efficiently in liquid phase for fast internal conversion, a large amplitude motion is required to reach this MECI, which is prohibited in the solid state and caused efficient AIE. This strategy is used to find experimentally that naphthalene analogues are also efficient AIE luminogens. The flexibility of alkyl chains on amino groups is also found to be important for allowed charge-transfer transition. Thus, three points (1. highly twisted N,N-dialkylamines, 2. substitution at the para positions 3. with flexible alkyl groups) were proposed for activation of small aromatic hydrocarbons.
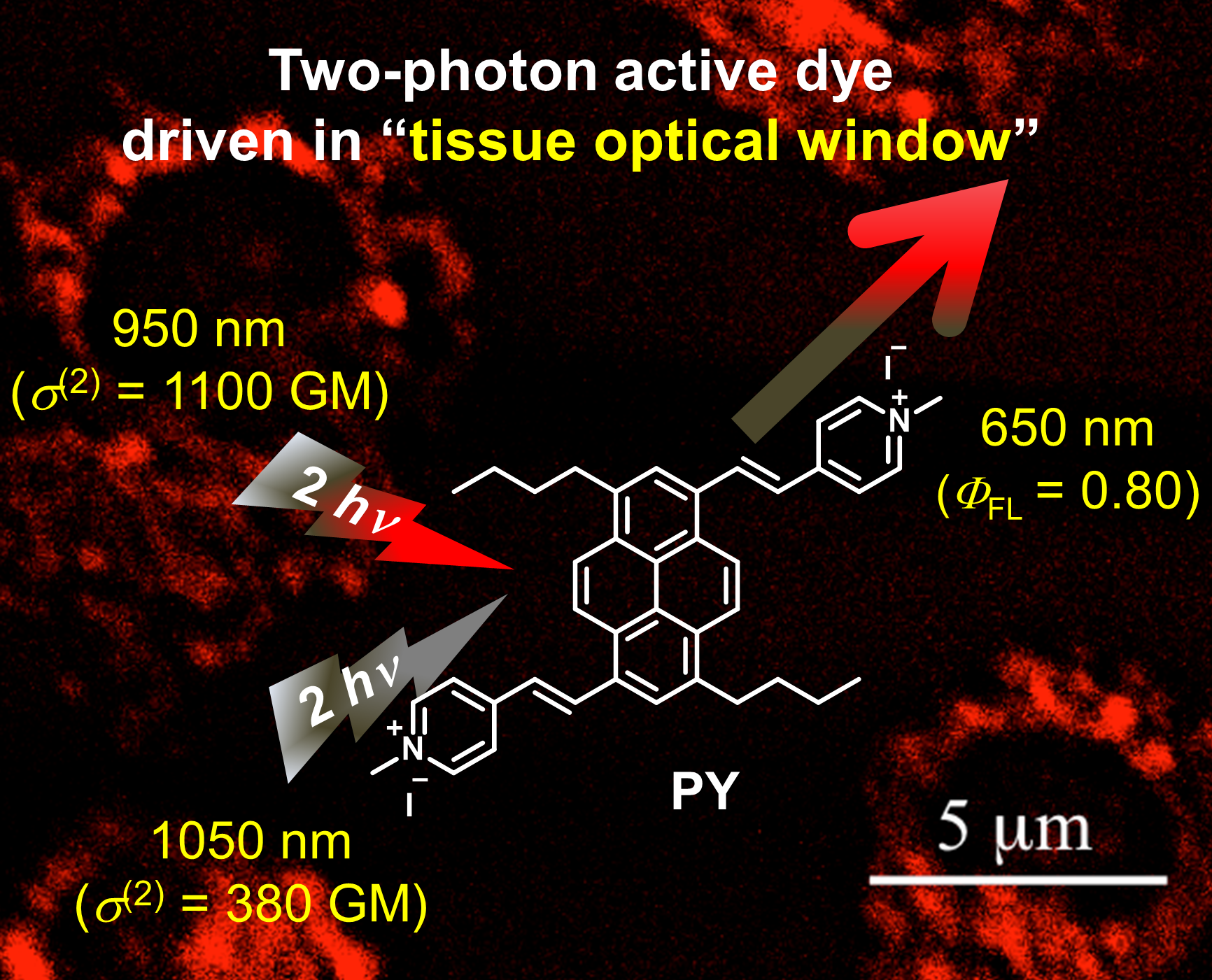
Novel pyrene-based two-photon active fluorescent dye efficiently excited and emitting in the ‘tissue optical window (650–1100 nm)’
J. Mater. Chem. B 2015 [DOI: 10.1039/C4TB01404A]
The development of two-photon (TP) active fluorophores remains an important issue. Dyes that can be excited and fluoresce efficiently in the ‘tissue optical window’ (650–1100 nm) are especially in demand to maximize the underlying performance of the two-photon fluorescence microscope (TPFM) as an advanced optical technique. Ideally, such dyes would be compatible with the 1050 nm femtosecond fibre laser, which has recently been developed as an inexpensive excitation source to make the TPFM technique universal. In this work, we designed and synthesized a novel pyrene-based acceptor-π-acceptor (A-π-A) dye, PY, which exhibited outstanding properties such as bright fluorescence (λem = 650 nm, ΦFL = 0.80) and a large two-photon absorption cross-section (1100 GM (1 GM = 10-50 cm4 photon-1 molecule-1) at 950 nm and 380 GM at 1050 nm) in the tissue optical window. In living mitochondria, PY provided more sensitive microscopic images than current dyes and showed great potential to be a building block of TP active fluorescent probes for the 1050 nm fibre laser. We believe the exceptional properties of PY will be extended to other fluorescent probes through further chemical modification.
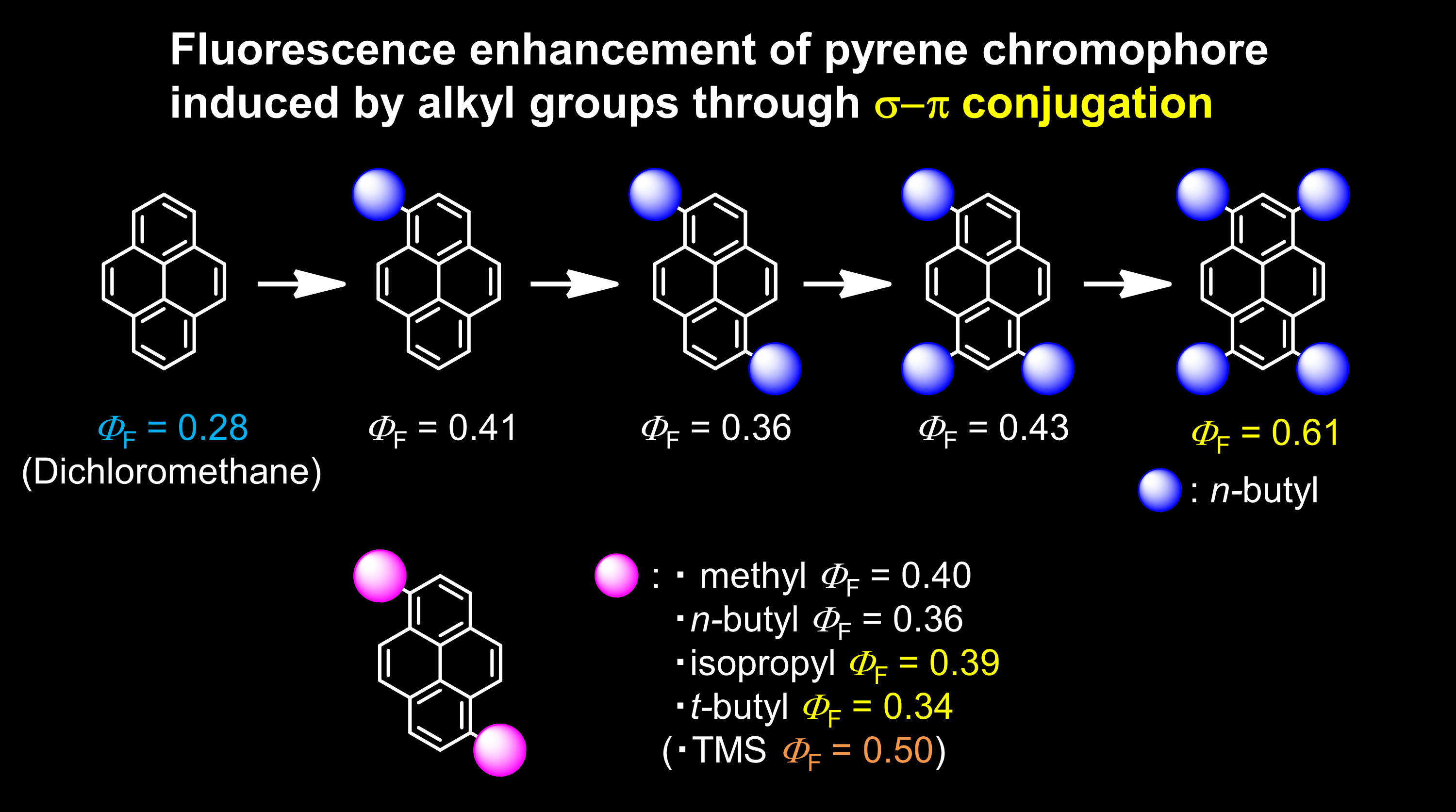
Fluorescence Enhancement of Pyrene Chromophores Induced by Alkyl Groups through sigma-pi Conjugation: Systematic Synthesis of Primary, Secondary, and Tertiary Alkylated Pyrenes at the 1, 3, 6, and 8 Positions and Their Photophysical Properties
J. Org. Chem. 2013 [DOI: 10.1021/jo400128c]
We have systematically synthesized 1-, 3-, 6-, and 8-alkyl substituted pyrene derivatives using the latest synthesis methods and investigated the effects of alkyl substitution on the photophysical properties of the pyrene chromophore. Like the trimethylsilyl group, which is known to enhance the fluorescence properties of some chromophores through sigma*-pi* conjugation, alkyl groups (primary, secondary, and tertiary) enhanced the fluorescence quantum yield of the pyrene chromophore through sigma-pi conjugation in most cases. While these enhancements in the fluorescence quantum yield were beyond expectations, the results were supported by absolute measurements. These results also indicate that ubiquitous alkyl groups can be used to tune the photophysical properties of the pyrene chromophore, as well as to improve the solubility or prevent aggregation. In other words, they can be used to develop new photofunctional materials.
Development of Laser Dyes to Realize Low Threshold in Dye-doped Cholesteric Liquid Crystal Lasers
Adv. Mater. 2010 [DOI: 10.1002/adma.201001046]
Highly efficient luminescence dyes based on pyrene and anthracene derivatives (see figure) are synthesized for liquid crystal dye lasers. The threshold value of one of the pyrene derivatives is as low as 1/20 that of the commonly used DCM in cholesteric liquid crystal (CLC) distributed feedback lasers. Good optical properties such as luminous efficiency and solubility in CLCs are important factors for realizing a low threshold.